Background
Multiple myeloma (MM) is a plasma cell malignancy characterized by the production of monoclonal light chains and immunoglobulin (M-protein), leading to impaired immune function, bone destruction, renal failure, and death. Existing and emerging MM therapies, including proteasome inhibitors (PI), immunomodulators (IMiDs), and monoclonal antibodies (mAB), delay progression and extend the lifespan but MM is incurable. However, more knowledge about patient experiences with treatments and their impact on everyday activities, including health-related quality of life (HRQOL) is needed for physicians and patients to make informed treatment decisions. This study aimed to gain a better understanding of the patient perspectives on treatment for relapsed and/or refractory multiple myeloma (RRMM).
Methods
This non-interventional, cross-sectional study involved semi-structured qualitative interviews with RRMM patients recruited from 6 U.S. clinics. Eligible RRMM patients (≥18 years) had at least 3 months of life expectancy, up to 6 prior lines of therapy, and at least one treatment regimen with a PI, IMiD, daratumumab, or an alkylating agent. Prior to conducting the patient interviews, a semi-structured qualitative interview guide was developed based on a literature review and formative/pilot discussions with MM expert clinicians (n=5) and patients (n=3). Within the interview guide, open-ended questions and targeted probes were used to facilitate discussions on topics including symptoms and impact of MM, mode of administration of treatment, tolerability, patients' perception of indicators of effectiveness and overall experience. Qualitative coding and content analysis were conducted using Atlas.ti v8.0.
Results
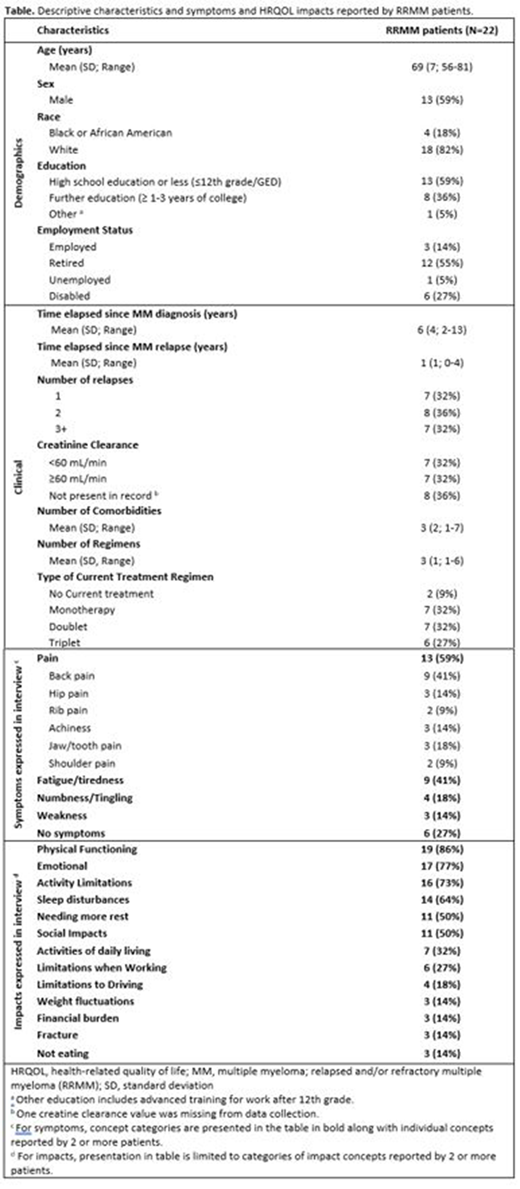
Demographic characteristics, symptoms, and HRQOL impacts reported by interview participants (N=22) are presented in Table. At enrollment, 59% of patients were using regimens containing dexamethasone, 36% daratumumab, 27% carfilzomib, 18% lenalidomide. Most common co-morbidities included hypertension (41%), respiratory illnesses (32%), and GERD (27%). More patients had experience using IV/injectable (41%) alone compared with oral therapy (18%) alone; 41% of patients reported having experience with both modes. Back pain (41%) and fatigue (41%) were the symptoms reported most frequently by patients; 27% of patients reported no symptoms (See Table). Among impact concept categories most commonly reported by patients, 86% experienced physical function limitations (e.g. difficulty walking or standing), 77% reported emotional impacts (e.g. worry/fear or sadness/depression), 73% reported activity limitations related to MM, and 64% reported sleep disturbances (see Table). Regarding treatment experiences, most patients (68%) perceived effectiveness of their treatment based on laboratory test results explained by their physician, while 41% based treatment effectiveness on symptom relief. When asked about treatment tolerability, most patients described experiencing gastrointestinal side effects (59%), fatigue (59%), sleep disturbances (32%) and allergic reactions (32%). Regarding mode of treatment administration and regimens, participants most commonly reported the overall length of each treatment day (68%), treatment interfering with daily activities (55%), and length of infusion (50%) as key elements of treatment burden.
Conclusions
This study provides key insights into the impact on HRQOL from symptoms and treatment burden as well as patient perspectives on treatment effectiveness, tolerability, and modes of administration. Considering the evolution and complexity of MM management, and increasing regulatory emphasis on patient-centeredness, patient-relevant factors should be incorporated into future evaluation of MM treatments and patient outcomes.
Bell:Millennium Pharmaceuticals, Inc.: Current Employment. Cherepanov:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Ginchereau Sowell:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Shah:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. McCarrier:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Research Funding. Hari:Amgen: Consultancy; BMS: Consultancy; GSK: Consultancy; Janssen: Consultancy; Takeda: Consultancy; Incyte Corporation: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal